Scientific Projects
(Colour-enhanced scanning electron micrograph, copyright by D. McCarthy and A. Cavanagh).
1) Metabolic biomarker identification in breast cancer
Diseases accompanied by strong metabolic disorders, like cancer show characteristic effects on cell turnover rate, activity of modifiying enzymes, DNA/RNA modifications and thus the resulting patterns of excreted modified nucleosides in biological fluids is altered. The observed elevated levels of certain ribonucleosides in the urine of cancer patients have established their potential as possible biomarkers in a non-invasive early diagnosis and therapy surveillance system.
- Collaboration partners: PD Dr. T. Erbes / Prof. E. Stickeler (Aachen), Dr. Marc Hirschfeld, Dr. S. Halbach, Prof. Dr. H. Neubauer (Düsseldorf), Prof. Dr. T. Fehm (Düsseldorf)
2) Regulation of plant biochemical pathways to investigate central metabolism, biosynthesis of secondary metabolites, signalling and stress response
Research projects with different woody (poplar, Douglas fir) and herbaceous (Arabidopsis) model plants, carried out at the CF Metabolomics, focus on questions of molecular physiology, plant physiology and ecophysiology. In particular, physiological response to abiotic stress (drought, flooding, cold) is studied by monitoring changes in single biomarkers and/or complex metabolite patterns. Gained insights into metabolic functioning help to tackle urgent challenges like efficient biomass production for energetic use, or tree and crop response to climate change.
- Collaboration partners: PD Dr. J. Kreuzwieser, Prof. Dr. A. Gessler (Zürich), Prof. M.-P. Laborie, Dr. H. Winter
3) Metabolomics of clinical significant mTOR dysregulations
Mechanistic target of rapamycin is a major regulator of metabolism, growth and survival and its dysregulation can cause severe clinical manifestations. We investigate metabolic alterations in two socioeconomic relevant diseases, i.e. diabetic nephropathy and autosomal dominant polycystic kidney disease. In both pathologies, mTORC1 is dysregulated via the tuberous sclerosis complex axis. We use different in vitro and in vivo model systems with genetic and exogenous disease triggers.
- Collaboration partners: Prof. Dr. C. Borner, Prof. Dr. G. Walz, Prof. Dr. T. Reinheckel, Prof. T. Huber (Hamburg)
4) Mitochondrial metabolomics: Metabolic response to genetic modifications in yeast
The mitochondrion has been described as the power house of the cell due to its major function-adenosine triphosphate (ATP) production, accompanied by electron transport chain (ETC), tricarboxylic acid cycle (TCA), along with other biological pathways. Defects in mitochondria are linked to human diseases such as cancer, Alzheimer’s disease and diabetes. These defects can cause physiological and metabolic alterations in mitochondria as well as in cytosol. Due to the huge metabolite pool in cytosol, it is difficult to address the origin of these alterations. Therefore, it is necessary to isolate mitochondria and cytosol to find out possible compartment-specific metabolic alterations, which are helpful in understanding the molecular mechanism of mitochondrial homeostasis in response to varies genetic mutations.
- Collaboration partner: Prof. Dr. N. Wiedemann
5) Disturbances of metabolic homeostasis in model Systems for nephronophthisis
Nephronophthisis is an autosomal recessive form of polycystic kidney disease and the leading cause of hereditary kidney failure in children and young adults. Nephronophthisis proteins (NPHP), such as Anks3 and Anks6, localise to the primary cilium, an important cellular sensing and signalling organelle, and lead to laterality defects and kidney failure in patients. Loss of NPHPs cause massive alterations on amino acid and nucleoside metabolism. In close collaboration with Prof. Walz, effects of NPHPs on DNA damage, proliferation as well as apoptosis were investigated to elucidate whether NPHPs are required for functional ciliary signalling and metabolic homeostasis.
- Collaboration partners: Prof. Dr. Gerd Walz, Dr. Vadym Budnyk
6) Biochemical and metabolic analysis and characterization of the asparagine metabolism in rhabdomyosarcoma
Oncogenic pathway activation alters key metabolic processes and enables growth and rapid production of new biomass in cancer cells. Cancer cells are dependent on certain metabolic processes, which may translate into specific vulnerabilities and open novel therapeutic opportunities. We will use a well-established in vitro/ in vivo platform of Ras-driven, murine rhabdomyosarcomas (RMS) to investigate the metabolic equilibrium of sarcoma cells and identify actionable vulnerabilities. Hettmer et al. have shown that RMS display asparagine dependency, which could be a weak point in the treatment of RMS (Hettmer et al. 2015). Therefore, we will characterize the asparagine metabolism and aim to identify new therapeutic targets.
- Collaboration partner: PD Dr. S. Hettmer
7) Preventing ischaemia-reperfusion injury - In vivo studies of novel molecular compounds
Pentameric C-reactive protein (pCRP), being a highly conserved pentameric protein, is a classical acute-phase protein during inflammation that acts as a precursor for the opsonizing monomeric C-reactive protein (mCRP). After transplantation, when tissue is revascularized it is prone to reperfusion which is being worsened by pCRP. After being activated by phospholipids from activated or ruptured membranes, pCRP dissociates into its monomers thus exacerbating the immune reaction.
Novel compounds have been synthesized which inhibit the phosphocholine induced dissociation and thus reduce formation of mCRP. These compounds have been tested in vitro and will be tested in vivo in Wistar Rats to determine bioavailability, pharmacokinetics and metabolism.
- Collaboration partners: Prof. Dr. S. Eisenhardt, Dr. Thiele, Prof. Dr. B. Breit
8) Autophagy in human keratinocytes:
Metabolic monitoring of LecB treated human keratinocytes
Pseudomonas aeruginosa is a gram negative and opportunistic pathogen, Its infections results in biofilm formation. The P. aeruginosa lectin LecB has been described to subvert several processes in the host cell but its impact on cellular metabolism has not been addressed so far.
Our screening reveals a dramatic impact of LecB on the amino acid and lipid metabolism. Specifically, whereas the amino acid levels are reduced, the lipid levels are increased upon treatment. As metabolism drives several essential cellular processes also during bacterial infections, investigating the metabolic host cell responses upon the bacterial lectin LecB may also provide a better understanding of skin infections and pave the way for new concepts in antibacterial therapies.
-
Collaboration partner: Prof. Dr. W. Römer, Dr. A. Landi
9) Identification of metabolic biomarkers of early and
late recurrent pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is one of the major causes of cancer-related mortality with 5-year overall survival below 5 %. Approximately 20 % of all PDAC patients are candidates for resection, whereas about 60 % of these patients experience local or metastatic recurrence within 12 months after curative surgery. Today, disease recurrence cannot be predicted with biomarkers at all. Many studies are aiming to identify biomarkers in patient blood or urine for prediction of disease recurrence.
With patient-derived organoid (PDO) cultures, PDAC of a variety of patients can be modeled in vitro and experimental findings can be correlated to the clinical course of the donating patients. With these model, current studies revealed a pivotal role of pancreatic stellate cells (PSCs) to support cancer cell metabolism and proliferation by secreting different metabolites.
- Collaboration Partner: Prof. U. Wittel, PD Dr. J. Kaiser
10) Biosynthesis and analysis of secondary metabolites from different bacterial strains and fungi as new therapeutic agents
Combinatorial biosynthesis is a procedure of molecular biology in which the combination of biosynthetical genes of different origins results in the formation of new natural products. The project is focused on metabolites with antibiotic activity isolated from actinomycetes or streptomyces which contains partially sugar moieties.
- Collaboration partners: Prof. Dr. S. Günther, Prof. Dr. R. Teufel, Prof. Dr. L. Heide (Tübingen), PD Dr. B. Gust (Tübingen)
11) Metabolomics to unravel molecular mechanisms involved in Parkinson's disease and aging
In a high-throughput metabolomics approach (fingerprinting & profiling) the effects of different PD agents on general metabolism in the model organism C. elegans was elucidated. The key metabolic hallmarks in cell damage development and cellular defence mechanisms were examined.
- Collaboration partners: Prof. Dr. S. Eimer
12) Metabolomics of Dexamethasone application in cochlear implants
Cochlear implants are hearing aids that provide a sense of sound for patients with impaired hearing. The auditory nerve innervating the cochlear is stimulated by electric signals. Though, it has been reported that due to inflammation the tissue surrounding the implant scars, thus increasing impedance and subsequently decreasing efficacy of the hearing aid. Analytics of metabolism and pharmacokinetics in regard of using different matrices have not been performed in detail.
Furthermore to the best of our knowledge, no data has been collected on the influence of glucocorticoids to the cellular homeostasis of inner ear cells which will be investigated in a cellular model using House Ear Institute-Organ of Corti 1 cells (HEI-OC1) cells.
- Collaboration partners: Prof Dr. B. Breit, Prof. Dr. S. Plontke (Halle), Dr. A. Liebau (Halle),
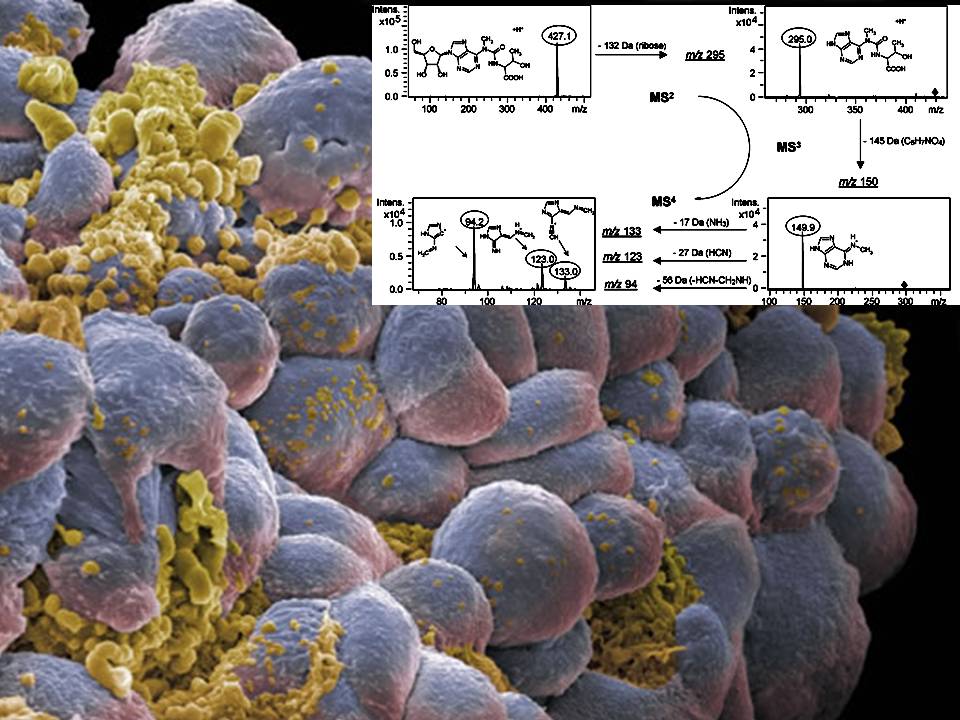
13) Analysis of modified nucleosides for biomarker discovery in renal cancers
Cancer cells have an increased RNA and DNA turnover due to their decoupling from regulatory mechanisms, controlling growth and survival. Modified nucleosides play an important role in different types of RNA and cannot be recycled and salvaged by common pathways. Therefore, they are excreted in greater amounts by tumor cells and ultimately accumulate in the urine, making them promising candidates for biomarkers. Targeted metabolomics approaches, like HPLC coupled to triple quadrupole mass spectrometry are used for identification. In this project, murine and human ccRCC models are used to identfy and characterize modified nucleosides as putative biomarkers for ccRCC.
- Collaboration partners: Prof. Dr. I. Frew, Prof. Dr. E. Neumann-Häfelin
14) Metabolic analysis of cerebrospinal fluid for the characterization of neurological disorders and neuroinflammation
Cerebrospinal fluid (CSF) represents the ideal biological matrix for the analysis of neurological disorders, since it is in direct contact with the central nervous system and therefor represents its metabolism closest. Many disease-related molecules accumulate in CSF, which enables metabolomics to decipher molecular mechanisms of diseases and to discover new biomarkers for neuroinflammatory, neurodegenerative or psychological disorders.
- Collaboration partners: Prof. Dr. B. Grimbacher
15) Metabolomics to investigate pathophysiological mechanisms in chronic myeloid leukemia and mastocytosis
This project employs mass spectrometry-based metabolomics to elucidate disease mechanisms in chronic myeloid leukemia (CML) and systemic mastocytosis. By analyzing metabolic alterations, we aim to identify biomarkers and therapeutic targets associated with disease progression and treatment resistance. The findings will contribute to a deeper understanding of myeloid malignancies and inform the development of targeted therapeutic strategies.
- Collaboration partners: Prof. Dr. Tilman Brummer, Dr. Sebastian Halbach
16) Metabolomic biomarker identification in ovarial cancer, endometrial cancer and endometriosis
Ovarian cancer, endometrial cancer, and endometriosis represent significant gynecological disorders with overlapping symptoms but distinct pathological mechanisms. Accurate and early diagnosis remains a clinical challenge, highlighting the need for specific and sensitive biomarkers. This project employs metabolomics-based approach to identify disease-specific metabolic signatures across these conditions focusing on modified nucleosides, endo and exo-metabolic profiling of cancer cell lines and patient samples.
- Collaboration partners: Prof. Dr. med Ingolf Juhasz-Böss, Dr. Clara Backhaus
17) Cystein-proteases inhibition in lung diseases
Using different in vitro and in vivo lung disease models, covering ARDS, SARS-CoV-2 infection and fibrosis, the efficacy of cathepsin inhibition in that conditions is studied. Pharmacodynamics, and -kinetics studies of the inhibitor are employed. Additionally, the impact on the cellular metabolome is investigated. In particular, the kynurenine pathway and eicosanoids are of interest.
- Collaboration partners: PD Dr. S. Fähndrich, Prof. Dr. D. Stolz, Prof. Dr. E. Papakonstantinou
18) Fecal metabolomics in correlation to the microbiome
The identification of metabolic differences between pathogenic and apathogenic H. pylori infections in the context of gastric cancer is evaluated in the PREDICT-HP study. Data will be correlated with metagenomics and serum markers. The impact of other pathogenic bacteria on the fecal metabolome is investigated using SImplified HUman MIcrobiome cultures (SiHuMi). The over-all aim of this project is to uncover biomarkers for fast identification of pathogens or critical events during pathogenesis.
- Collaboration partners: Prof. Dr. G.A. Häcker, Dr. T. Badr
19) Elucidation of conformational changes by RNA modifications
To date, over 100 different RNA modifications are known, and many of them are associated with diseases like cancer, neurological diseases or infections. With an orthogonal approach, we study the conformational changes of small RNA molecules induced by modifications like methylation, acetylation, and others. For that, ion mobility mass spectrometry and nanopores are used.
- Collaboration partners: Dr. Tobias Ensslen, Dr. Sebastian Gutsch
20) AutoMeta - Automated sample preparation for metabolomics analyses
Medical treatments are often ineffective or cause side effects in many patients. Metabolomics offers real-time insights into individual health and drug responses. However, manual sample preparation limits reliability. Therefore, we are developing automated microfluidic chips to streamline this step, making metabolomics more accurate and accessible for personalized medicine.
- Collaboration partners: Prof. Dr. R. Teufel, Dr. T. Hutzenlaub, Dr. N. Klatt
21) Comparison of machine learning models and explainability techniques for biomarker identification in CML: Focus on modified nucleosides
Chronic myeloid Leukemia (CML) is a rare disease that afflict around 1.000 people in Germany every year. In this work, we employ machine learning techniques on sparse data, and have focused on modified nucleosides to identify biomarkers. Unlike most models that fail to converge with small amounts of data, our machine learning models are successful at extracting features by SHAP. The methods are then compared in the results.
- Collaboration partners: M.Sc. G. Islamoglu
22) Micro-ESI-needle array for spatial metabolomics analyses
In cooperation with the Rapp group, micro-needle arrays are developed which can sample cells in a raster-based manner. These needle arrays also function as ESI-emitter with separate electric control of each needle after mounting to an interface. Thus, each fraction of the array can be sequentially sampled to the mass-spectrometer allowing for a
spatially resolved metabolome and proteome analysis.
- Collaboration partners: Prof. Dr. B. Rapp, Prof. Dr. P. Huesgen, Prof. Dr. W. Römer
23) Correlation analyses Metabolome – Transcriptome, advanced bioinformatics
Several researchers not only acquired metabolome data but also other omics data sets like transcriptome. To assist the integration of multi-omics data, we have a strong cooperation with the Günther group. Also, simulated metabolic pathways could be validated in our facility, including binding or pharmacokinetics studies.
- Collaboration partners: Prof. Dr. S. Günther
24) Structural analysis of natural compounds in Namibian medicinal plants
Inflammatory diseases, autoimmune disorders, and cancer are appearing more frequently and at earlier stages than ever before. Accordingly, there's a significant need for anti-inflammatory and cancer therapeutics. To address this, we analyze promising Namibian medicinal plants for their bioactive compounds.
- Collaboration partners: Prof. Dr. med. Roman Huber, Prof. Dr. Davis Mumbengegwi, Prof. Dr. Robin Teufel
25) Structural and semiquantitative analysis of natural compounds in grapevine extract
Like most monocultures, viticulture requires large quantities of plant protection products, such as fungicides. Even in organic viticulture, these are used in form of inorganic copper salts, which, unlike organic molecules, accumulate in the soil and thus degrade soil quality. A newer approach is the use of grapevine extract, which contains fungicidal active compounds. We analyze these compounds and their behavior in the field.
- Collaboration partners: Prof. Dr. Stefan Rensing, Prof. Dr. Andreas Bechthold
26) Cluster of Excellence Furture Forests
The CF Metabolomics is involved in the following sub-areas of the Cluster of Excellence Future Forests through joint projects starting January 2026:
Theme A1: Data-driven prediction of effective hydro-climatic and biogeochemical conditions in complex forest ecosystems (Lead: Christen, Lang, Contributors: Orth, Stahl, Weiler, Kammerer, Seifert, Puhlmann): Impact of forests’ climate modulation on the dynamics of soil organic matter and the pro-vision of plant nutrients (WP3)
Theme A2: Responses and adaptations of organisms to novel environmental conditions (Lead: Heer, Werner; Contributors: Biedermann, Blumenstein, Hartman, Kammerer, Kowallik, Kleine-Vehn, Ragni, Seifert): Trees will exposed to hot droughts and subsequent herbivore and pathogen infections (WP1) and monitor their response at the molecular level (WP2) by considering epigenetic and gene expression changes, as well as adjustments at the stage of protein, at the level of metabolite production.
Theme A3: Risks and adaptation options from trees to forest stands and land-scapes (Lead: Bauhus, Seifert; Contributors: Albrecht, Hanewinkel, Hauck, Kammerer, Kattenborn, Lang, Munteanu, Schnabel.) WP1. Analysis of stress patterns that lead to mortality in mature trees.
27) Identification of mTORC2-regulated bioactive signaling molecules in stress response
mTORC2 signaling plays a critical role in the regulation of lipid metabolism. We aim to identify bioactive lipid signaling molecules that act downstream of mTORC2 to modulate transcription factors involved in stress resistance in the nematode Caenorhabditis elegans.
- Collaboration partners: Prof. Dr. R. Baumeister., Dr. T. Heimbucher, Dr. G. Wu